-40%
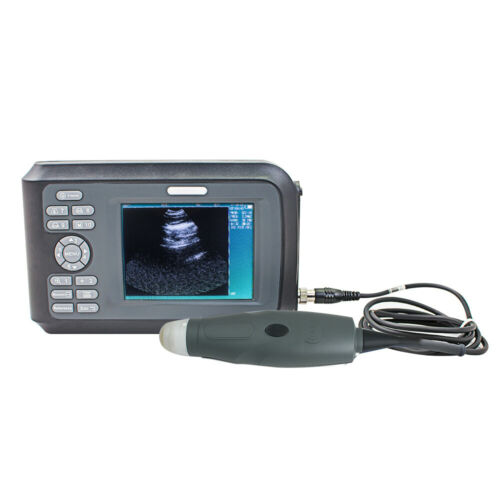
Digital Medical Ultrasound Scanner With 3.5MHZ Convex Probe 3D Image *DHL Ship*
$ 633.07
- Description
- Size Guide
Description
Product OverviewDigital Medical Ultrasound Scanner With 3.5MHZ Convex Probe 3D Image Pregnancy
Detail
SKU: EDA500306---RUS-9000A2
Features For Ultrasound Scanner
High-frequency beam-former to ensure best resolution of the image
8steps for TGC and digital over-all Gain grasp the high-quality image
Whole-course real-time continuous dynamic focusing and aperture obtains high-resolution and picture in picture in
Super professional software packages to have the scanner widely used in Obstetrics Gynecoloyg,Urology, Cardiology ,Small parts.
Easy for operators with the comfortable application and professional software package.
It is the best choice for clinical diagnosis,because it makes clinic diagnostic more convenient and effective.
Specification For Ultrasound Scanner:
Screen Size
10.4 inch
Display Mode
B, B+B, B+M, M, 4B
Image gray scale
256 scale
Cine loop
≥500 Frame
Image storage
64 Frame
Scale Angle
Adjustable
Scan depth
40mm~240mm
Image flip
Up/down,left/right,black/white
Image Process
GAMA,Image Smoothen.THI,Histogram,Zoom
Focus
Focus Number,Focus position,Focus space
Measure
Distance,circumference,area,volume,heart,GAFW,EDD
Character display
Date,clock,name,PID,age,sex,hospital name,doctor
Output report
4 types
USB port
USB2.0
Power consumption
200VA
Size
1220mm×665mm×468mm
Packing list:
1×Main Unit
1×3.5MHZ convex probe
1×Lilon battery
1×Power adapter(AC110V~240V,2A,50/60Hz)
1×Operation Manual
Shipping Policy
Payment Mathord
Returns Policy
About Feedback
Contact US
Shipping Policy
1. We accept PAYPAL payment only and ship to your eBay address.
2. Item will be shipped within 1 business days after received a verified payment.
3. Shipped by air mail which normally takes 7-14 business days to USA, 12-25 business days to other countries.
4. Delivery Time upon purchase Locations
>>>Australia, South East Asia and Asia
15-18 working days
>>>United Kingdom, United States and Canada
8-21 working days
>>>Europe, South America, Middle-East Asia
16-28 wrokging days
>>>Italy and Brazil (because of strict custom inspection)
28-56 working days
Payment Mathord
1. PayPal payment accepted only.
2. Items will be shipped to your eBay address. Please make sure it is correct.
3. Payment must be received 4 days after auction ended.
Returns Policy
1. Customer satisfaction is our top goal. We believe our items are so outstanding. All products are quality checked. They are new and in good condition when shipped to our customers. We are convinced you will be happy with your Purchase.
2. If product is defective or damage upon arrival, or wrong product shipped, please contact us immediately. Returns accepted within 14 days of delivery date and item must be in original new condition, not worn or altered in any way with attached tags & wrap. Otherwise deal is final. Return shipping must be paid by buyer.
3. Please contact us first if you have any problems/questions/concerns. We will be happy to resolve any issues you may have in a cordial and friendly manner.
4. We appreciate your Postive Feedback, and will do the same in return. DO NOT leave negative feedback without first communication with us. Please allow max 2 business days for us to response.
About Feedback
When you satisfied with our product and services please leave us positive feedback.
If a problem occurs, contact us immediately with any email request. We promise we will give you the satisfied solution. :)
Contact US
The seller’s name: Alice
City:Beijing
State: Beijing
Country: China
Telephone number: 86-18305265146
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health