-40%
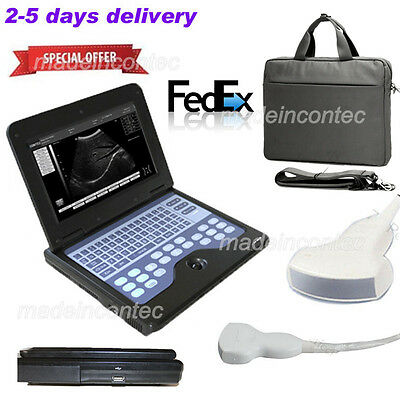
Portable laptop machine Digital Ultrasound scanner,3.5 Convex probe Newest CE US
$ 580.27
- Description
- Size Guide
Description
Portable laptop machine Digital Ultrasound scanner,3.5 Convex probe Newest CE USIntroductions
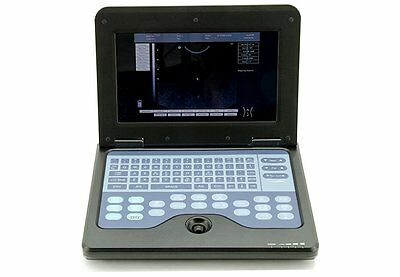
The notebook type CMS600P2 is a full digital B-Ultrasound diagnostic system. It adopts embedded operating system, which greatly optimizes the product performance. The system is taken more conveniently for its high effective data processing ability, pop-up menu and keyboard design. It includes rich measuring software packages that satisfy the clinic diagnostic need fully.
Main Features
◆The device which adopts linux embedded operating system, has well compatibility, transplantable character, and flexible expansibility.
◆It adopts advanced full digital beam-forming, real time dynamic aperture, real time dynamic receiving apodization, real time dynamic receive focusing, frame correlation, modern image processing technology etc., which improves image quality.
◆The system can be taken conveniently for notebook type, built-in battery, high integration of unit, small volume, light weight.
◆It adopts touch type folding keyboard, flexible and shortcut trackball operation, which greatly quicken the test speed.
◆Neat and convenient image managing function which can print and export report.
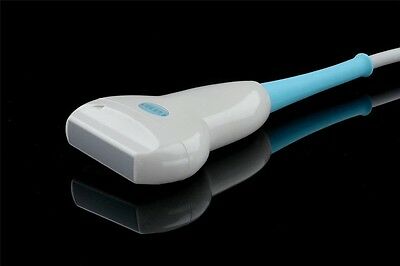
◆It includes rich measuring software packages:obstetrics, gynecology, cardiology, urology, small parts. Many probes can be chosen, and wide application can satisfy the clinic diagnostic need fully.
◆Near gain, far gain and total gain can be adjustable alone.
◆Possess external memory function.
◆Can be connected to the video printer, inkjet printer, laserjet printer.
Main Performance
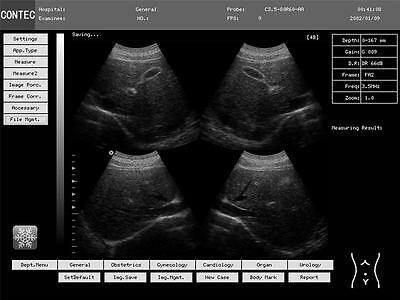
Display mode:B,2B,4B,B/M,M
Image gray scale:256
Monitor:10.1 inch TFT LCD
Display resolution :1024*600
Lateral resolution:2 mm (near field) 4 mm (far field)
Axial resolution:2 mm (near field) 2 mm (far field)
Dead zone:≤3 mm
Display depth:≤240 mm
Horizontal geometric position precision:≤5%
Vertical l geometric position precision:≤5%
Zoom:0.9, 1.0, 1.1, 1.2, 1.3, 1.5, 2.0
Cine loop:600 frames
Image storage:2048 frames
Body mark:43
Focus:The number and position can be adjustable.
Assistant tool:Puncture guide and histogram
Obstetrics, gynecology, cardiology, small parts, urology etc. can generate reports automatically.
Measurement:Distance, circumference, area, volume,angle, ratio, slope etc.
Support Chinese, English language interface display and input.
The management interface menu, Chinese and English operating systems, image processing default and a key function of optimization.
Measurement formula can be set up differently according to the different race.
Comment:Date, time, name, hospital,number, frame rate, depth, gain, dynamic range, frame correlation, frequency etc.
Image processing:Controllable frame correlation, gamma correction, histogram
Image conversion:Up and down, left and right, black and white
Tissue harmonic imaging
Screen comments function in both Chinese and English
Support USB storage
Update software though USB
Interface:USB,Video,VGA
Standard Configuration
3.5 MHz convex probe, 2.0 MHz~5.0 MHz
Optional Configuration
6.5 MHz endo-vaginal probe, 5.0 MHz~8.5 MHz
7.5 MHz linear probe, 5.0 MHz~10.0 MHz
Physical Identity
Dimension:292 mm*232 mm*45 mm
Weight:2.3 kg (include probe)
Qualification
CE
Buy Safe Product!!
■
The following FDA Disclaimer is required for all eBay listing in
Healthcare category and is included for REFERENCE:
■
The sale of this item may be subject to regulation by the U.S.
Food and Drug Administration and state and local regulatory agencies.
■
If the item is subject to FDA regulation,We will verify your status
as an authorized purchaser of this item before shipping of the item.
■
The
model
is registered on the Australian Register of Therapeutic
Goods (ARTG)
with the code 197923,
and certified by FDA
510K Approved of America and CE,TUV of Europe
On May-05-17 at 00:34:09 PDT, seller added the following information:
Only English user guide,if you need any other language,please contact us!