-40%
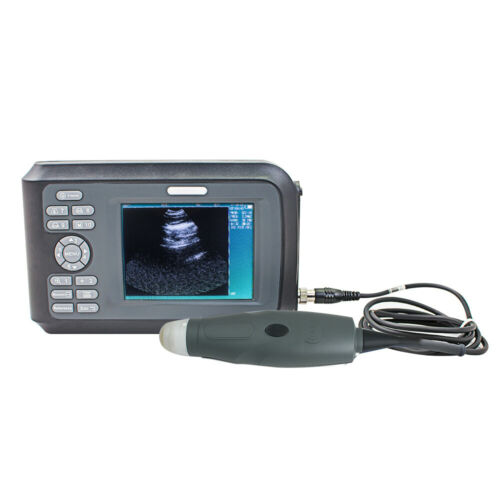
Portable Laptop Machine ultrasound diagnostic Scanner+3.5MHZ Convex Probe US FDA
$ 580.27
- Description
- Size Guide
Description
Portable laptop machine ultrasound diagnostic Scanner+3.5MHZ Convex Probe US FDAStandard Configuration
3.5 MHZ Convex Probe
I
ntroductions
The notebook type CMS600P2 is a full digital B-Ultrasound diagnostic system. It adopts embedded operating system, which greatly optimizes the product performance. The system is taken more conveniently for its high effective data processing ability, pop-up menu and keyboard design. It includes rich measuring software packages that satisfy the clinic diagnostic need fully.
Main Features
◆The device which adopts linux embedded operating system, has well compatibility, transplantable character, and flexible expansibility.
◆It adopts advanced full digital beam-forming, real time dynamic aperture, real time dynamic receiving apodization, real time dynamic receive focusing, frame correlation, modern image processing technology etc., which improves image quality.
◆The system can be taken conveniently for notebook type, built-in battery, high integration of unit, small volume, light weight.
◆It adopts touch type folding keyboard, flexible and shortcut trackball operation, which greatly quicken the test speed.
◆Neat and convenient image managing function which can print and export report.
◆It includes rich measuring software packages:obstetrics, gynecology, cardiology, urology, small parts. Many probes can be chosen, and wide application can satisfy the clinic diagnostic need fully.
◆Near gain, far gain and total gain can be adjustable alone.
◆Possess external memory function.
◆Can be connected to the video printer, inkjet printer, laserjet printer.
Main Performance
Display mode:B,2B,4B,B/M,M
Image gray scale:256
Monitor:10.1 inch TFT LCD
Display resolution :1024*600
Lateral resolution:2 mm (near field) 4 mm (far field)
Axial resolution:2 mm (near field) 2 mm (far field)
Dead zone:≤3 mm
Display depth:≤240 mm
Horizontal geometric position precision:≤5%
Vertical l geometric position precision:≤5%
Zoom:0.9, 1.0, 1.1, 1.2, 1.3, 1.5, 2.0
Cine loop:600 frames
Image storage:2048 frames
Body mark:43
Focus:The number and position can be adjustable.
Assistant tool:Puncture guide and histogram
Obstetrics, gynecology, cardiology, small parts, urology etc. can generate reports automatically.
Measurement:Distance, circumference, area, volume,angle, ratio, slope etc.
Support Chinese, English language interface display and input.
The management interface menu, Chinese and English operating systems, image processing default and a key function of optimization.
Measurement formula can be set up differently according to the different race.
Comment:Date, time, name, hospital,number, frame rate, depth, gain, dynamic range, frame correlation, frequency etc.
Image processing:Controllable frame correlation, gamma correction, histogram
Image conversion:Up and down, left and right, black and white
Tissue harmonic imaging
Screen comments function in both Chinese and English
Support USB storage
Update software though USB
Interface:USB,Video,VGA
Standard Configuration
3.5M Convex Probe
Optional Configuration
3.5 MHz Micro-convex probe, 2.0 MHz~5.0 MHz
7.5 MHz linear probe, 5.0 MHz~10.0 MHz
6.5 MHz endo-vaginal probe, 5.0 MHz~8.5 MHz
Physical Identity
Dimension:292 mm*232 mm*45 mm
Weight:2.3 kg (include probe)
Buy Safe Product!!
■
The following FDA Disclaimer is required for all eBay listing in
Healthcare category and is included for REFERENCE:
■
The sale of this item may be subject to regulation by the U.S.
Food and Drug Administration and state and local regulatory agencies.
■
If the item is subject to FDA regulation,We will verify your status
as an authorized purchaser of this item before shipping of the item.
■
The
model
is registered on the Australian Register of Therapeutic
Goods (ARTG)
with the code 197923,
and certified by FDA
510K Approved of America and CE,TUV of Europe.
Only English user guide,if you need any other language,please contact us!
Powered by SoldEazy